The Future
of Itch Relief
The future of itch relief
Numelvi is registered for the treatment of pruritus associated with allergic dermatitis including atopic dermatitis in dogs and for the treatment of inflammation and clinical manifestations of atopic dermatitis in dogs.

Selective
Preferentially targets the JAK1 enzyme, the primary driver of itch and inflammation in allergic dermatitis

Well Tolerated
For dogs and puppies from 6 months old with tablets from 2 kg* in weight

Simple
Easy once-daily dosing from the first dose, to be given at or around mealtimes.

Effective
Significant reduction in itch from 2 hours after the first dose
*A benefit-risk assessment should be made by the responsible veterinarian for dogs less than 3 kg as safety has not been investigated in these dogs.
Skin and Allergic Dermatitis
Skin is the most affected body system in dogs1,2,3
Skin infections and otitis externa are common in dogs2,4
Skin is the #2 reason dogs receive veterinary care3
Itch is the most common clinical sign in dogs4,5,6

Allergic dermatitis5,6


Atopic dermatitis
Environmental allergens, like house dust mites, mould, plants (pollens) and also food

Food-responsive dermatitis
Proteins like beef, dairy, chicken and wheat

Flea allergy dermatitis
Proteins and other substances in flea saliva
Other
Insect-bite, contact, etc.
Prevalence of allergic dermatitis
Difficult to determine as studies vary in:
- Diagnostic and inclusion criteria
- Geography
- Type of veterinary practice enrolling cases
Prevalence varies due to overlap in clinical signs
^ More than one presumed cause possible

The Science of Selectivity

Cytokines use JAK enzymes to stimulate intracellular signaling pathways.

JAK1 is the primary driver of itch and inflammation.

First-generation JAK inhibitors target the JAK enzymes with little to no selectivity.8

High selectivity for JAK1 helps minimize interference with the beneficial functions of other JAKs.
Numelvi is at least 10x more selective for JAK1 over the other JAKs.†
† Over the other JAK enzymes in in vitro assays.
Prevalence of allergic dermatitis
Difficult to determine as studies vary in:
- Diagnostic and inclusion criteria
- Geography
- Type of veterinary practice enrolling cases
Prevalence varies due to overlap in clinical signs
Why is selectivity important
- JAK1 is the primary driver of itch and inflammation
- Erythropoeitin and Thrombopoeitin are mediated exclusively by JAK2
- Inhibition of JAK2 impacts cytokines involved in haematopoeisis and immune response
- First-generation JAK inhibitors target the JAKs with little to no selectivity

Janus Kinase enzymes
Janus Kinase enzymes are a family of four receptor-associated tyrosine kinase enzymes located inside cells. Cytokines use JAK enzymes to stimulate immune cells.
- JAK1
- JAK2
- JAK3
- Tyrosine kinase 2 (TYK2)
JAKs participate in many processes in the body including:
- Itch and inflammation
- Host defense (immune response and regulation)
- Production and differentiation of blood cells

Mechanism of Action

Rapidly effective
- Significant reduction in itch 2 hours after first dose
- For the treatment of pruritus associated with allergic dermatitis including atopic dermatitis in dogs
- For the treatment of inflammation and clinical manifestations of atopic dermatitis in dogs
Proven safety and efficacy
- At least 10X more selective† for JAK1, the primary driver of itch and inflammation
- High selectivity for JAK1 minimises interference with beneficial functions of other JAKs
- Allows adequate response to vaccination when dosed at 3X maximum recommended dose
- Well tolerated in dogs from 6 months of age
Simple and convenient
- Once daily dosing from day 1
- Broadest age and weight range – the only JAK inhibitor for use in dogs from 6 months of age
† Over the other JAK enzymes in in vitro assays.
Find out what the studies say about Numelvi7
Atinvicitinib inhibits cIL-31-induced itch in dogs more than oclacitinib9
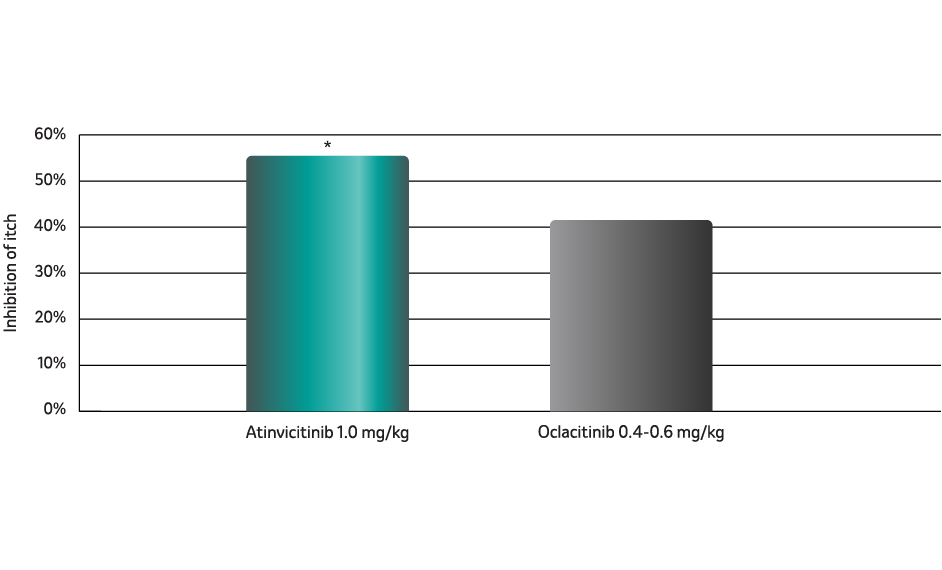
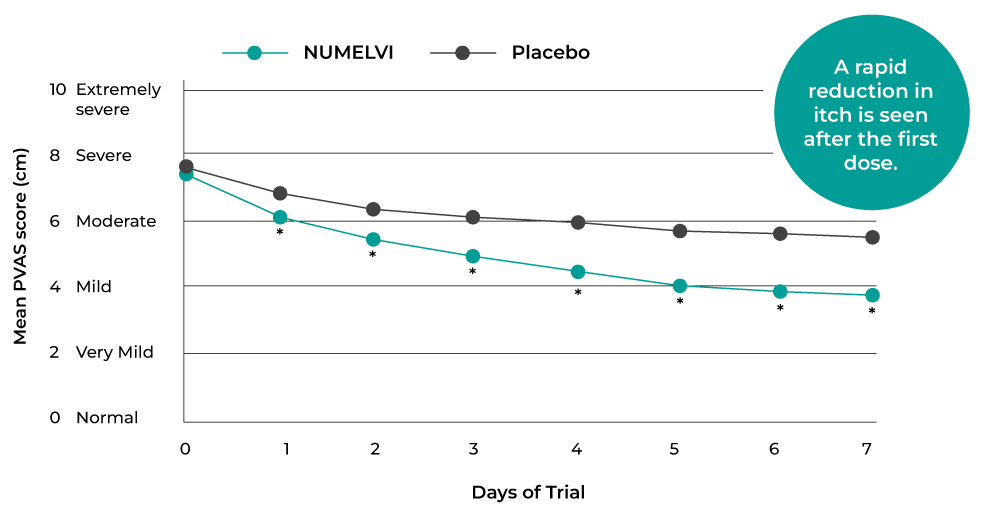
Significant reduction in itch from the first dose10
Clinically relevant reduction in itch in >81% of dogs within 1 week1
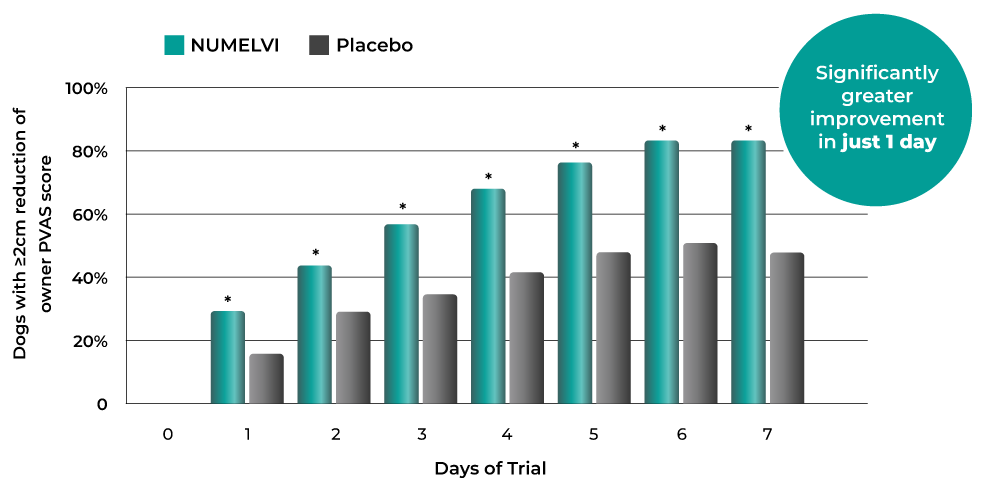
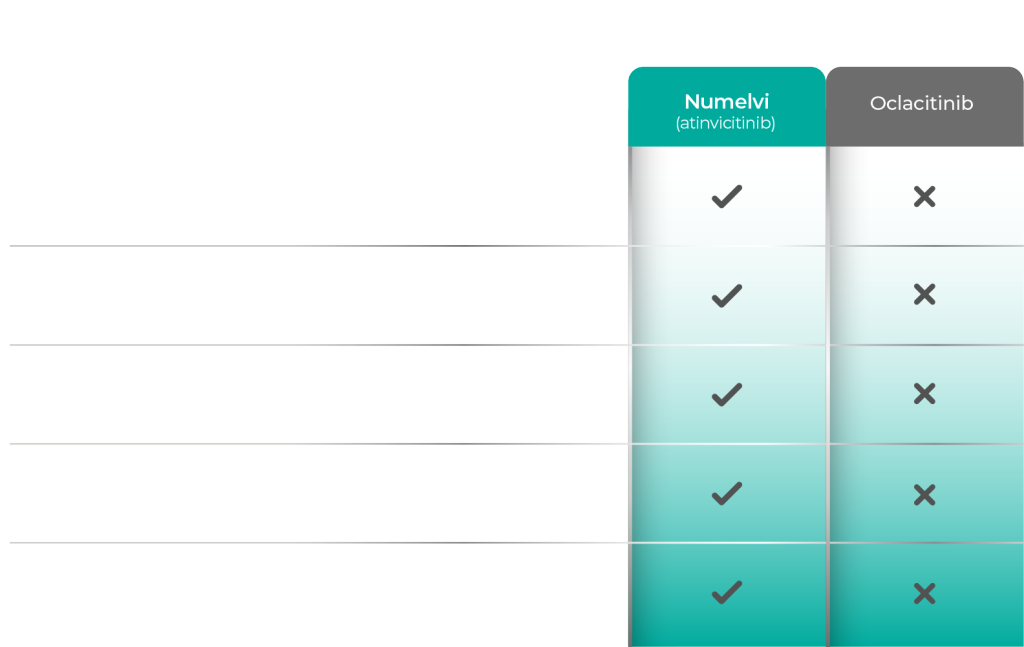
Compare Numelvi With the Competition
Simple dosing
The average recommended dose is 1.0 mg atinvicitinib per kg body weight orally once daily at or around mealtime.

Available in 4 Tablet Strengths for Simple Dosing

Pack images are for illustration purposes
Easy Dosing With Most Dogs Requiring 1 Whole or One-half Tablet
The average recommended dose is 1.0 mg of atinvicitinib per kg body weight orally once daily at or around mealtime.
Simplicity and Convenience
Numelvi makes managing itch seamless.
Simple for Veterinarians
- Option for all dogs and puppies from 6 months of age
- Easy dosing helps owner compliance
Simple for Dog Owners
- Once-daily dosing from the start
- Consistent relief with consistent dosing
- Reassuring safety profile
Allergic Dermatitis Can Be a Chronic Condition
Some types of allergic dermatitis are chronic, while others can be seasonal. Either way, managing the itch and inflammation with your veterinarian is important.
Finding relief will not only improve your dog’s well-being – but also your own peace of mind.

Significant reduction in itch from 2 Hours after first dose9
Once-daily dosing starts relieving itch from day 1 with continued improvement thereafter7
† Over the other JAK enzymes in in vitro assays.
Resources
Access these materials to learn more about allergic dermatitis and how best to care for your itchy dog.
Have a question about Numelvi?
Contact an MSD Animal Health RepresentativeThere’s More to Learn About the Future of Itch Relief
Please fill out the form below to request a call from an MSD Animal Health representative and join our Numelvi mailing list.
Schering-Plough Animal Health Ltd(trading as MSD Animal Health) will collect, record and use your name, phone number, postcode and email address and other information you provide in this form (“personal Information”), for only as long as is necessary for the purposes of contacting you with updates in relation to MSD Animal Health products’,unless we are required or permitted by law to hold the information for a longer period). We may disclose your personal information to organisations who assist us and our affiliated companies (which may be located in other countries including the United States, United Kingdom, Singapore, South America, Poland, China or India). If you would like to know more about our privacy policy, including how to access and seek correction of the personal information MSD holds about you, how to complain about a breach of the New Zealand Privacy Principles, and how MSD handles such complaints, please go to or to https://www.msdprivacy.com/nz/en/index.html, or contact our Privacy Officer at: Level 3, 123 Carlton Gore Road, Newmarket, Auckland, 1023, T 523 6000, msd_privacy_office@msd.com NZ-NUM-251000028
1. Wiles BM, Llewellyn-Zaidi AM, Evans KM, et al. Canine Genet and Epidemiol. 2017;4:8. 2. O’Neill DG, Church DB, McGreevy PD, et al. PLoS One. 2014;9(3):e90501. 3. Hill PB, Lo A, Eden CAN, et al. Vet Record. 2006;158:533-539 4. Miller J, Simpson A, Bloom P, et al. J Am Anim Hosp Assoc. 2023;59(6):255-284. 5.Hensel P, Santoro D, Favrot C, et al. BMC Vet Res. 2015;11:196. 6. Mueller R. Atopic dermatitis and food-responsive dermatosis. In: Jackson HA, Marsella R, eds. BSAVA Manual of Canine and Feline Dermatology, 4th ed. BSAVA; 2021:76-81. .7. Jirjis et al The Second-generation Janus Kinase 1Selective Inhibitor Atinvicitinib: A Safe and in Dogs with Allergic Dermatitis. Presented at European Veterinary Dermatology Congress; Bilbao, Spain; 11-13 September 2025. 8. Gonzales AJ, Bowman JW, Fici GJ, et al. J Vet Pharmacol Ther. 2014;37(4):317-3. 9. Kowalski et al “The Second-Generation Janus Kinase Inhibitor Atinvicitinib Significantly Reduces Pruritus 2-4 Hours After Dosing Dogs in a Canine Interleukin-31 Model” European Veterinary Dermatology Congress; Bilbao, Spain:11-13 September 2025.
10. Jirjis et al The Second-generation Janus Kinase 1Selective Inhibitor Atinvicitinib: A Safe and in Dogs with Allergic Dermatitis. Presented at European Veterinary Dermatology Congress; Bilbao, Spain; 11-13 September 2025. 11. European Medicines Agency. Numelvi: PUAR – Product Information. 2025. 12. European Medicines Agency. Apoquel: PUAR – Product Information & Assessment Report. 2025.
Numelvi, ACVM No: A012149, is AVAILABLE ONLY UNDER VETERINARY AUTHORISATION